AMES, Iowa, April 02, 2019 (GLOBE NEWSWIRE) -- NewLink Genetics Corporation (NASDAQ:NLNK) today announced results from a Phase 2 study of NLG207, a nanoparticle formulation of the topoisomerase 1 inhibitor camptothecin, conducted in conjunction with The GOG Foundation, Inc. (GOG-3008), in patients with refractory ovarian cancer. The data were presented in a poster session at the American Association for Cancer Research (AACR) Annual Meeting 2019 in Atlanta, Georgia.
"We are encouraged by the responses observed in this Phase 2 trial of NLG207 in combination with weekly paclitaxel for patients with recurrent ovarian, fallopian tube or primary peritoneal cancer, with good results seen in the cohort of platinum resistant and refractory patients,” said Linda R. Duska, MD, Professor of Obstetrics and Gynecology at the University of Virginia School of Medicine. “Equally encouraging is how well tolerated this regimen was in women who have already had multiple lines of therapy. The results from this trial demonstrate the potential for NLG207 as a component of a treatment regimen for women with recurrent ovarian cancer, especially for those resistant to platinum therapy, an area of unmet need.”
Background:
NLG207 (formerly CRLX101), a nanoparticle-drug conjugate (NDC) composed of a cyclodextrin-based polymer backbone linked to camptothecin, a topoisomerase 1 inhibitor, was previously observed to have single agent activity in both preclinical1 and clinical studies.2 A previous Phase 2 trial evaluating NLG207 administered as monotherapy enrolled 29 patients with ovarian, fallopian tube, or primary peritoneal cancer, 22 of which were classified as platinum resistant. Of these 22 patients, 19 were evaluable for efficacy with follow-ups using computerized tomography (CT) scans performed every two cycles. Of these 19 evaluable patients, 17 (74%) demonstrated a net tumor reduction and three (16%) achieved durable partial responses per RECIST.2
In the study reported on today, NLG207 was administered in combination with paclitaxel to patients with recurrent ovarian, fallopian tube, or peritoneal cancer in a Phase 1b/2, single-arm, open label expansion study. Thirty patients were enrolled, and all received at least one cycle of treatment: 3 patients received the lower lead-in Phase 1b dosing schedule of 12 mg/m2 i.v. (every two weeks) plus weekly paclitaxel 80 mg/m2 i.v. (three weeks on, one week off) and 27 patients received the recommended Phase II dose NLG207 15 mg/m2 i.v. (every two weeks) plus weekly paclitaxel 80 mg/m2 i.v. (three weeks on, one week off). The primary objective of the study was overall response rate (ORR = complete response [CR] + partial response [PR]) per RECIST 1.1. Secondary objectives included evaluation of progression-free survival (PFS), PFS at 6 months (PFS6), and duration of response (DOR) in patients with recurrent platinum resistant cancer, and safety.
Results:
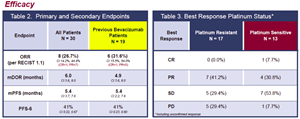
- N = 30 patients, with all completing at least one cycle (27/30 at Phase 2 dosing schedule; 3/30 at lower lead-in Phase 1b dosing schedule)
- 57% received ≥ 3 prior therapies
- ORR 26.7% (8/30) confirmed, including one CR
- Median DOR was 6.0 months
- Median PFS was 5.4 months
- PFS6 was 41%
- Patients with platinum resistant cancer: N = 17
- ORR 23.5% (4/17) confirmed
- Best response (including unconfirmed) 41.2% (7/17), all PRs
- Stable disease (SD) 29.4% (5/17)
- Patients with platinum sensitive cancer: N = 13
- ORR 30.8% (4/13) confirmed and best response
- SD 53.8% (7/13)
- Most common treatment related grade 3 / 4 adverse events: decreased neutrophil count 43% (13/30), anemia 10% (3/30), hematuria 7% (2/30)
Conclusions:
NLG207 is a potentially best-in-class topoisomerase 1 inhibitor with demonstrated antitumor activity in recurrent ovarian cancer, including cancer resistant to platinum therapy. The combination therapy of NLG207 plus weekly paclitaxel was well tolerated in heavily pretreated patients with most adverse events consistent with those observed for paclitaxel as a single agent. Prior single agent activity observed in both preclinical and clinical studies, along with Phase 2 results in combination with paclitaxel reported here, support further investigation of NLG207 as a single agent and in combination treatment regimens for patients with recurrent ovarian, fallopian tube or primary peritoneal cancers, particularly those which are platinum resistant.
“We were excited by the single agent activity of NLG207 observed in prior trials and are encouraged by the Phase 2 data presented today at AACR that support the potential for this drug candidate to improve upon current therapies in ovarian cancer, particularly given its favorable safety profile,” said Charles J. Link, Jr, MD, Chairman and Chief Executive Officer of NewLink Genetics. “We wish to thank the patients, their families, and the investigators who participated in this trial as we continue our efforts to better the lives of individuals suffering from cancer.”
Abstract CT151 (Poster 17) entitled, A phase II study of NLG207 (formerly CRLX101) in combination with weekly paclitaxel in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer, Duska, L., et al, was presented during the poster session entitled, “Phase II-III Clinical Trials: Part 1,” in Exhibit Hall B, Poster Section 16 from 8:00 a.m. - 12:00 p.m. ET. The poster presentation is also available on NewLink Genetics’ website in the “Investors & Media” section, under “Posters & Publications.”
| 1 |
Pham E, et al. Clin Can Res.2014; 21(4); 808–18. |
| |
Lin CJ, et al. Oncotarget.2016 Jul 5;7(27):42408-42421. |
| |
|
| 2 |
Pham E, et al. Clin Can Res. 2014; 21(4); 808-818. |
| |
Weiss GJ, et al. Invest New Drugs. 2013;31:986-1000. |
| |
Krasner CN, wet al. J Clin Oncol. 2014; 32:Abstract 5581. |
About NLG207
NLG207 (formerly CRLX101) is an investigational nanoparticle-drug conjugate (NDC) composed of a cyclodextrin-based polymer backbone conjugated to camptothecin, a topoisomerase 1 inhibitor. NDCs enhance drug delivery to tumors where gradual payload release inside cancer cells augments antitumor activity while reducing toxicity. Topoisomerase 1 inhibitors are a class of drugs that modify DNA damage responses in cancer cells. NewLink Genetics is evaluating NLG207 in a series of clinical trials in advanced refractory ovarian cancer patients.
About NewLink Genetics Corporation
NewLink Genetics is a clinical stage biopharmaceutical company focusing on developing novel oncology product candidates to improve the lives of patients with cancer. NewLink Genetics' IDO pathway inhibitors, indoximod and its prodrug, NLG802, are immuno-oncology drug candidates designed to harness multiple components of the immune system to combat cancer. NewLink Genetics’ nanoparticle drug candidate, NLG207, conjugated to camptothecin, a topoisomerase 1 inhibitor is under development to combat refractory malignancies. For more information, please visit www.NewLinkGenetics.com and follow us on Twitter @NLNKGenetics.
About The GOG Foundation, Inc.
The GOG Foundation, Inc. (GOG Foundation) is an independent international non-profit organization with the purpose of promoting excellence in the quality and integrity of clinical and basic scientific research in the field of gynecologic malignancies, including cancers that arise from the ovaries, uterus, cervix, vagina, and vulva. The mission of the GOG Foundation is to conduct clinical and translational research that positively impacts women through the prevention and treatment of gynecologic malignancies.
This press release contains forward-looking statements of NewLink Genetics that involve substantial risks and uncertainties. All statements contained in this press release are forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “will be,” "may," “appear to,” “has potential to,” “look forward to,” “are designed to,” or the negative of these terms or other similar expressions, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about results of NewLink’s clinical trials for product candidates and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that NewLink Genetics makes due to a number of important factors, including those risks discussed in "Risk Factors" and elsewhere in NewLink Genetics' Annual Report on Form 10-K for the year ended December 31, 2018 and other reports filed with the U.S. Securities and Exchange Commission (SEC). The forward-looking statements in this press release represent NewLink Genetics’ views as of the date of this press release. NewLink Genetics anticipates that subsequent events and developments will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing NewLink Genetics' views as of any date subsequent to the date of this press release.
Investor & Media Contact:
Lisa Miller
Director of Investor Relations
NewLink Genetics
515-598-2555
lmiller@linkp.com
Source: NewLink Genetics Corporation
A photo accompanying this announcement is available at http://www.globenewswire.com/NewsRoom/AttachmentNg/b646c1e3-a67d-48a3-8393-21562fb38c2f

Source: NewLink Genetics Corporation