NewLink Genetics Announces Positive Updated Phase 1 Data with Indoximod Plus Radio-Immunotherapy for Pediatric Patients with DIPG Presented at ISPNO 2018
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20180702005283/en/
(Graphic: Business Wire)
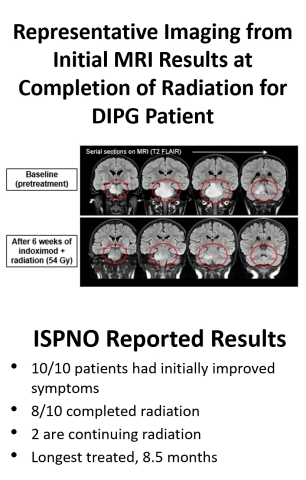
Data were presented on ten newly diagnosed DIPG patients, all of whom had initiated therapy at the time of this assessment. All (10/10) demonstrated initial symptomatic improvement. Eight of ten had completed radiation, with the remaining 2 of 10 patients continuing radiotherapy. While a subset of the patient cohort developed inflammatory and other adverse symptomology, a common occurrence in this patient population, these symptoms were actively managed. Currently, 9/10 patients remain on study, with the longest time on study of 8.5 months. These data include more mature follow-up on the 6 patients previously presented at AACR 2018.
“These data continue to demonstrate the potential for indoximod plus radiochemotherapy as a combination treatment regimen which may improve disease related symptoms for these pediatric patients with an otherwise dire prognosis,” said Dr. Theodore S. Johnson, M.D., Ph.D., Associate Professor of Pediatrics at Augusta University, lead investigator for the trial. “We remain encouraged and look forward to additional data as the study proceeds.”
This DIPG cohort is a subset of
About Diffuse Intrinsic Pontine Glioma (DIPG)
Diffuse intrinsic pontine glioma, or DIPG, is a rare, aggressive brain
tumor found in the brain stem that almost exclusively affects
children. Every year in
1
About Indoximod
Indoximod is an investigational, orally available small molecule targeting the IDO pathway. The IDO pathway is a key immuno-oncology target involved in regulating the tumor microenvironment and immune escape. Indoximod is being evaluated in combination with treatment regimens including chemotherapy, radiation, checkpoint blockade and cancer vaccines across multiple indications such as AML, DIPG and melanoma.
About NewLink Genetics Corporation
NewLink Genetics is a clinical stage biopharmaceutical company focusing on discovering, developing and commercializing novel immuno-oncology product candidates to improve the lives of patients with cancer. NewLink Genetics' IDO pathway inhibitors are designed to harness multiple components of the immune system to combat cancer. For more information, please visit www.newlinkgenetics.com and follow us on Twitter @NLNKGenetics.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements
of NewLink Genetics that involve substantial risks and uncertainties.
All statements contained in this press release are forward-looking
statements within the meaning of The Private Securities Litigation
Reform Act of 1995. The words "may," “appear to,” “has potential to,”
“look forward to,” or the negative of these terms or other similar
expressions are intended to identify forward-looking statements,
although not all forward-looking statements contain these identifying
words. These forward-looking statements include, among others,
statements about results of NewLink’s clinical trials for product
candidates and any other statements other than statements of historical
fact. Actual results or events could differ materially from the plans,
intentions and expectations disclosed in the forward-looking statements
that NewLink Genetics makes due to a number of important factors,
including those risks discussed in "Risk Factors" and elsewhere
in NewLink Genetics' Annual Report on Form 10-K for the year
ended December 31, 2017 and other reports filed with the U.S. Securities
and Exchange Commission (
View source version on businesswire.com: https://www.businesswire.com/news/home/20180702005283/en/
Source:
Investor:
NewLink Genetics
Lisa Miller, 515-598-2555
Director
of Investor Relations
lmiller@linkp.com
or
Media:
LaVoieHealthScience
Sharon
Correia, 617-374-8800, ext. 105
VP, Integrated Communications
scorreia@lavoiehealthscience.com

